Angle Closure Glaucoma
Angle closure glaucoma – Written by Associate Professor Monisha Esther Nongpiur, MD, PhD.
What is angle closure glaucoma?
Glaucoma is a group of eye diseases that can damage the nerve at the back of the eye called the optic nerve, resulting in the gradual and irreversible loss of vision. Untreated, it can lead to blindness. Glaucoma is classified according to the configuration of the anterior chamber angle (the front part of the eye between the cornea and iris that is mainly responsible of drainage of fluid from the eye) into two major types: open-angle and angle-closure (Figure 1).
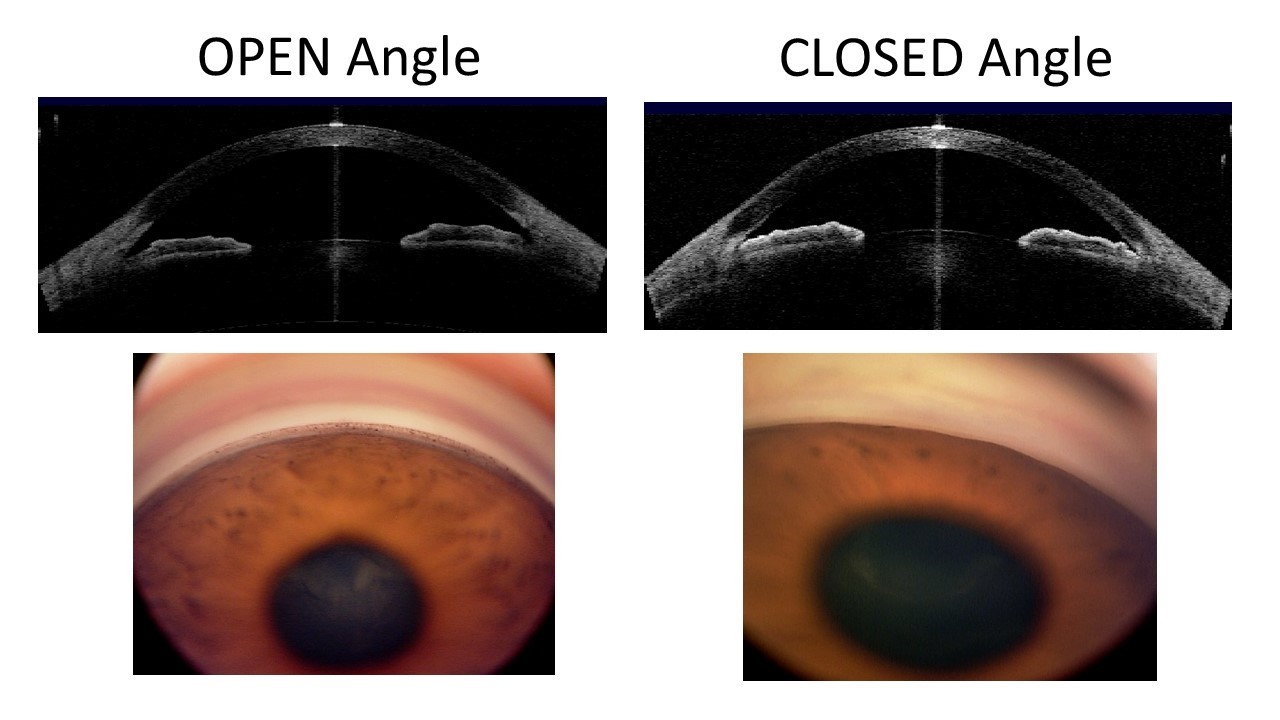
Primary angle-closure glaucoma (PACG), as the name suggests, is characterised by an obstruction of the drainage angle by the iris (the coloured part of the eye). Blockage of the drainage angle can lead to elevation of the eye pressure and damage to the optic nerve. Angle closure glaucoma is also called closed-angle glaucoma or narrow-angle glaucoma.
It is important to differentiate between open-angle and angle-closure glaucoma because the treatment approaches differ. Eyes with angle closure glaucoma may require additional procedures compared to eyes with open angle glaucoma.
This overview will cover the public health problem of angle closure glaucoma, the different stages and mechanism of angle closure glaucoma, who are at risk of developing angle closure glaucoma, its signs and symptoms, how it is diagnosed, its management options, as well as whether it can be prevented.
How many people have angle closure glaucoma?
Compared to primary open angle glaucoma (POAG), which is more common in Caucasians and Africans [1,2], PACG is more prevalent in Asia. It is estimated that the number of people (aged 40 to 80 years) with PACG worldwide will increase to 32 million by 2040, with Asia accounting for 76% of the worldwide PACG cases [3]. This high figure has important public health implications since visual loss, once established, cannot be reversed. PACG is also a more aggressive disease, and carries a greater risk of disease progression and development of glaucoma blindness compared to POAG. The economic burden is further increased since visual impairment due to glaucoma is associated with a significant impact on daily activities including limitations of physical and social abilities [4], even at earlier stages of the disease, well before blindness develops.
What are the different stages of angle closure glaucoma?
Angle closure glaucoma is a disease spectrum which commences with the earliest stage called primary angle closure suspect (PACS), followed by primary angle closure (PAC) and culminates in PACG [5]. This classification scheme that is accepted by the World Glaucoma Association, postulates the natural process and progression of the disease by identifying three separate conceptual stages to its development:
- PACS in whom eyes have a narrow drainage angle with the absence of signs of trabecular damage such as PAS and/or elevated eye pressure, and with no optic nerve damage.
- PAC with the same angle findings as PACS, but with the presence of signs of trabecular damage, and with no optic nerve damage.
- PACG, where there is a narrow drainage angle combined with structural and functional damage to the optic nerve consistent with glaucoma.
The common feature in all three stages is the presence of narrow drainage angles, also known as occludable angles. Primary angle closure disease (PACD) is a collective term, which includes all the three stages of the disease spectrum from PACS, to PAC, to PACG.
How does angle closure glaucoma develop?
‘Pupil block’ is considered to be the primary mechanism for angle closure glaucoma development. It occurs when the contact between the iris and the lens at the level of the pupil increases the resistance to the flow of fluid (aqueous humour) into the anterior chamber. This causes forward bowing of the peripheral iris (‘iris bombe’) and closure of the angles, thereby impeding the outflow of the aqueous resulting in an increase in the eye pressure (Figure 2).

The other mechanisms for angle closure include plateau iris and lens-induced. Plateau iris syndrome results from a large and/or anteriorly positioned ciliary body which alters the position of the peripheral iris. The condition is characterised by the presence of persistent angle closure despite the presence of a patent iridotomy (will be described below under the management of angle closure glaucoma section). A large and anteriorly placed lens worsens angle crowding in eyes with angle closure.
Who is at risk of developing angle closure glaucoma?
Angle closure glaucoma is typically more common in women [6,7], people with hypermetropia (long-sightedness) [8] and in older persons [6,7]. It is also more common in persons of Asian ethnicity, for example Chinese [9,10] and in Inuits (Eskimos) and in those with a family member with angle-closure glaucoma [11, 12]. A possible reason for the gender differences is the significantly narrower anterior chamber angles of female eyes compared to that of men [13]. With age, there is a gradual shallowing of the anterior chamber and narrowing of the angles [13,14].
Although the racial differences in the prevalence of angle closure is well-established, however, the mechanisms underlying these differences are still not fully understood. Furthermore, the overall eyeball size (axial length) of eye with angle closure is not only small, but the front part of the eye (anterior segment) is also more crowded as the lens is thicker and more anteriorly placed (Figure 3) [8, 15, 16].

With the advent and advances in imaging technology, several novel imaging-based parameters have been found to be associated with an increased risk of angle closure (Figure 4). These include smaller dimensions of the anterior chamber for example the width, area and volume [17, 18], thicker iris with a greater iris area [19], and also a larger proportion of the lens that is located in the anterior chamber (lens vault) [20].

A number of genetic variants have also been found to be associated with PACG. They probably contribute to the disease via different pathways and mechanism [21, 22].
What are the signs and symptoms of angle closure glaucoma?
Aptly called the ‘Silent thief of sight’, glaucoma is largely asymptomatic until late in the disease course when irreversible damage to the optic nerve has occurred. In the earlier stages of the disease, peripheral vision is affected with relative sparing of the central vision. But as the disease progresses, central vision is affected and it may even lead to blindness if left untreated.
There is, however, one type of angle closure glaucoma, the acute primary angle closure (APAC) that is associated with acute symptoms. It is an ocular emergency with very high eye pressure and can occur at any stage of the spectrum of disease. It is characterized by severe eye pain, blurred vision with haloes, redness, headache, nausea, and vomiting [23]. Immediate reduction of eye pressure is warranted to prevent irreversible damage to the optic nerve.
If you experience any of these signs and symptoms, schedule an appointment with an eye health professional to get your eyes checked. It is also important to note that the development of eye conditions may even start before symptoms appear, which makes going for regular and timely eye checks that much more essential.
How will angle closure glaucoma progress?
Disease progression in glaucoma is not uncommon and occurs even despite treatment. However, the rate of progression varies among patients – some with more aggressive disease progress rapidly whereas others progress relatively slowly. Older age and greater optic nerve damage at presentation were associated with a higher likelihood a more rapid rate of progression in PACG.
How is angle closure glaucoma diagnosed?
Gonioscopy is the current gold standard technique for evaluation of the anterior chamber angles and to diagnose angle closure glaucoma. The procedure is performed at the slit-lamp and involves placing a special contact lens (gonioscopy lens) in the eye (with or without a coupling fluid depending on the lens used) (Figure 5). A beam of light from the slit-lamp is then used to illuminate the angles. This allows the examiner to determine if the angles are open or closed, and also to assess the presence and extent of peripheral anterior synechiae (PAS), which are anterior adhesion of peripheral iris to the anterior chamber angle.

In addition to gonioscopy, the other routine tests done to diagnose glaucoma include:
- Measurement of the eye pressure with a tonometer which is usually performed at the slit-lamp
- Examination of the structure of the optic nerve (clinically or by imaging)
- Evaluation of the function of the optic nerve through visual field test
How do you manage angle closure glaucoma?
The goal of angle closure glaucoma management is to prevent or slow progression of the disease and to maintain the overall quality of life of the patient. Most patients with glaucoma maintain useful vision if detected early and treatment initiated. Treatment modalities include laser, medications and surgery.
Laser peripheral iridotomy (LPI)
LPI is the initial treatment option for eyes with angle closure glaucoma as it relieves pupil block. LPI is done in asymptomatic eyes with angle closure (PACS, PAC, PACG) to prevent an acute angle closure attack, and also as a treatment if a patient is having an acute attack.
During the procedure, a laser is used to make a tiny hole in the iris. The procedure provides an alternative pathway for the aqueous to flow from the posterior to the anterior chamber, bypassing the pupil.
Data from recent randomized controlled trials on PACS subjects [24, 25], observed modest but significant benefit of prophylactic LPI in PACS, the earliest stage of the angle closure disease spectrum. They recommend observation without LPI is a reasonable option for asymptomatic PACS.
Additionally, early lens extraction has been recommended as an alternative to LPI as an initial treatment for PAC and PACG [26].
Other laser procedures such as laser iridoplasty, selective laser trabeculoplasty (SLT) and cyclophotocoagulation are also available as potential therapeutic options for the management of angle closure.
Medical treatment
The goal of medical treatment in the management of glaucoma is to reduce the eye pressure. The medications work by either reducing the aqueous production and/or increasing the aqueous outflow. The medications for PACG are similar to that of open angle glaucoma, comprising prostaglandin analogues, beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors.
Surgical treatment
Surgical treatment is usually indicated when there is inadequate control of the eye pressure and disease progression, despite medical or laser treatment. The different surgical options include cataract extraction, trabeculectomy, combined cataract extraction and trabeculectomy and glaucoma drainage devices (tube-shunt).
In trabeculectomy, an alternate outflow pathway of the aqueous from the anterior chamber to the subconjunctival space is made by creating a small flap in the sclera (white part of the eye) and a bubble-like pocket in the conjunctiva called a bleb. Fluid drains out of the eye through the flap and into the bleb where it is absorbed by tissues around the eye, thereby lowering the eye pressure. In the procedure for the glaucoma drainage devise, a small implant or tube is placed in the eye to drain the aqueous fluid in order to lower the eye pressure.
Deciding on the most appropriate surgical procedure in a patient with PACG is complex, and involves consideration of several factors such as the severity of glaucoma, magnitude of eye pressure, the presence and severity of cataract.
Can angle closure glaucoma be prevented?
As glaucoma is generally asymptomatic, therefore regular eye check-ups are the best form of prevention so that early signs of the disease or its progression can be picked up and necessary treatment initiated.
DISCLAIMER: THIS WEBSITE DOES NOT PROVIDE MEDICAL ADVICE
The information, including but not limited to, text, graphics, images and other material contained on this website are for informational purposes only. No material on this site is intended to be a substitute for professional medical advice, diagnosis or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
References
- Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA 1991;266(3):369-74.
- Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992;99(10):1499-504.
- Tham YC, Li X, Wong TY, et al. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014.
- Freeman EE, Munoz B, West SK, et al. Glaucoma and quality of life: the Salisbury Eye Evaluation. Ophthalmology 2008;115(2):233-8.
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002;86(2):238-42.
- Wong TY, Foster PJ, Seah SK, Chew PT. Rates of hospital admissions for primary angle closure glaucoma among Chinese, Malays, and Indians in Singapore. Br J Ophthalmol 2000;84(9):990-2.
- Seah SK, Foster PJ, Chew PT, et al. Incidence of acute primary angle-closure glaucoma in Singapore. An island-wide survey. Arch Ophthalmol 1997;115(11):1436-40.
- Sihota R, Gupta V, Agarwal HC, et al. Comparison of symptomatic and asymptomatic, chronic, primary angle-closure glaucoma, open-angle glaucoma, and controls. J Glaucoma 2000;9(3):208-13.
- Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol 2001;85(11):1277-82.
- Foster PJ, Oen FT, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 2000;118(8):1105-11.
- Lowe RF. Primary Angle-Closure Glaucoma. Family Histories and Anterior Chamber Depths. Br J Ophthalmol 1964;48:191-5.
- Leighton DA. Survey of the first-degree relatives of glaucoma patients. Trans Ophthalmol Soc U K 1976;96(1):28-32.
- Alsbirk PH. Anterior chamber depth in Greenland Eskimos. I. A population study of variation with age and sex. Acta Ophthalmol (Copenh) 1974;52(4):551-64.
- Foster PJ, Alsbirk PH, Baasanhu J, et al. Anterior chamber depth in Mongolians: variation with age, sex, and method of measurement. Am J Ophthalmol 1997;124(1):53-60.
- George R, Paul PG, Baskaran M, et al. Ocular biometry in occludable angles and angle closure glaucoma: a population based survey. Br J Ophthalmol 2003;87(4):399-402.
- Aung T, Nolan WP, Machin D, et al. Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol 2005;123(4):527-32.
- Nongpiur ME, Sakata LM, Friedman DS, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology 2010;117(10):1967-73.
- Wu RY, Nongpiur ME, He MG, et al. Association of narrow angles with anterior chamber area and volume measured with anterior-segment optical coherence tomography. Arch Ophthalmol 2011;129(5):569-74.
- Wang B, Sakata LM, Friedman DS, et al. Quantitative iris parameters and association with narrow angles. Ophthalmology 2010;117(1):11-7.
- Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology 2011;118(3):474-9.
- Vithana EN, Khor CC, Qiao C, et al. Genome-wide association analyses identify three new susceptibility loci for primary angle closure glaucoma. Nat Genet 2012;44(10):1142-6.
- Khor CC, Do T, Jia H, et al. Genome-wide association study identifies five new susceptibility loci for primary angle closure glaucoma. Nat Genet 2016;48(5):556-62.
- Lee KY, Rensch F, Aung T, et al. Peripapillary atrophy after acute primary angle closure. Br J Ophthalmol 2007;91(8):1059-61.
- He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet 2019;393(10181):1609-18.
- Baskaran M, Kumar RS, Friedman DS, et al. The Singapore Asymptomatic Narrow Angles Laser Iridotomy Study (ANA-LIS): 5 year results of a Randomized Controlled Trial. Ophthalmology 2021.
- Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet 2016;388(10052):1389-97.
Tools Designed for Healthier Eyes
Explore our specifically designed products and services backed by eye health professionals to help keep your children safe online and their eyes healthy.
