Central Serous Retinopathy
Central serous retinopathy – Written by Dr. Stacy Chan Hsiao Lan, Bachelor of Optometry (N.Z), Doctorate in Optometry (U.K)
What is central serous retinopathy?
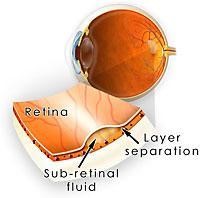
The retina is the the light sensitive tissue at the back of the eye that contains millions of light sensitive cells allowing vision. The macula is the area of the retina that allows us to enjoy central and colour vision. In central serous retinopathy (CSR), fluid accumulates between the retinal layers in the macula, thereby affecting one’s central vision. CSR is also known as central serous chorioretinopathy (CSCR) as the choroid (layer that supplies the retina with nutrients) is believed to be involved in the development of CSR.
This overview will briefly outline how many people are affected by CSR, who is at risk of developing CSR, how CSR develops, its signs and symptoms, how it is detected, as well as some insights into its treatment options.
How many people are affected by central serous retinopathy?
There is limited data reporting how common CSR is globally. A study in the United States of America (Olmsted County) estimated that 5.8 per 100,000 individuals suffer from CSR [1], while a study in India reported that 1.7% of their population are affected by CSR [2].
In Japan, an annual incidence rate of approximately 0.27% in the general population aged 30 years or older was reported in a recent (2021) study [3]. In other Asian countries, such as South Korea [4] and Taiwan [5], similar annual incidence rates of approximately 0.17% and 0.21% has been reported in the general population.
Who is at risk of central serous retinopathy?
CSR is considered among other major eye conditions such as diabetic retinopathy, branch retinal vein occlusion, and age-related macular degeneration that affects the retina [6]. CSR typically affects young or middle aged adults (25 to 50 years old) [7] and is 6 times more common in men than women [1].
There is a strong association between CSR and the “Type-A” personality pattern (personality traits of having a competitive drive, a sense of urgency and an aggressive nature) [8]. Recent psychological stress is also an important trigger for the development of CSR [9].
Although the link between CSR, personality traits, and stress are still poorly understood, higher levels of circulating stress hormones particularly corticosteroids and catecholamine are found in those who have CSR [10].
As such, people with Cushing syndrome (characterized by abnormally high levels of cortisol) and pregnant women are also at risk of CSR due to increased levels of related corticosteroids. People undergoing treatment with systemic corticosteroids such as cortisone can also suffer from CSR.
A strong association between CSR and sleep disturbances has also been established [10]. Other risk factors such as hypertension, kidney disease, genetic predisposition, and bacterial infection with Helicobacter pylori may play a role in the development of central serous retinopathy [10].
How does central serous retinopathy develop?
The mechanism of disease development of CSR remains unknown. However, it has been suggested that choroidal dysfunction in the choroid (pigmented layer at the back of the eye that nourishes the retina) triggers retinal pigment epithelial dysfunction and leakage of fluid in CSR [13].
Categories of central serous retinopathy
CSR can be classified as follows:
Acute
A single episode of CSR occurring in a person’s lifetime with sub-retinal detachment that resolves spontaneously within 1 to 6 months from the onset of symptoms.
Prolonged or non-resolving
CSR that lasts longer than 6 months but spontaneously resolves within 12 months, impacting vision.
Recurrent
Episodes of acute CSR following a previous episode of acute CSR with complete resolution of sub-retinal detachment.
Inactive
People with a history of acute CSR but without sub-retinal detachment at the time of evaluation.
What are the common signs and symptoms of central serous retinopathy?
- Vision is blurred in one eye
- Dark spot in central vision (scotoma)
- Distorted vision
- Problems with colour vision
- Reduced contrast
If you experience any of these signs and symptoms, schedule an appointment with an eye health professional to get your eyes checked. It is also important to note that the development of eye conditions may even start before symptoms appear, which makes going for regular and timely eye checks that much more essential.
How does an eye health professional confirm that you have central serous retinopathy?

Upon examination of the retina, the macula region displays shallow serous detachment. Due to the raised retina, the focal length of the eye is decreased, therefore slightly or moderately affecting the vision of the affected eye. As a result, the affected eye has more hypermetropia (far-sightedness) than before.
When swelling of the macula is suspected, a test called an Amsler grid is performed to identify the presence of central scotoma (blind spot) and/or image distortion, if any. The Amsler grid could also help to localize the area of visual field affected.

In addition to the tests conducted during a routine eye check, a scan of the eye is performed using an equipment called an optical coherence tomography (OCT). An OCT scan provides high quality and resolution of the retinal layers. In CSR, features such as a bubble of central serous retinal detachment, retinal pigment epithelial (layer of pigment cells forming part of the retina) detachment, and retinal pigment epithelial abnormalities can be observed in an OCT scan.

Treatment options for central serous retinopathy
Monitoring and reassurance
Monitoring the progression or regression of CSR and reassurance is usually the best treatment course for people with acute CSR as the sensory retina usually reattaches and vision improves within 3 to 4 months [16]. However, approximately 20 to 30% of patients will develop one or more recurrences of acute CSR and 5% will develop chronic CSR [10].
It is also crucial for people with CSR to suspend or replace any current medications containing steroids with an alternative.
Laser treatment
Fluid within the sub-retinal space can also be reduced using laser photocoagulation being applied close to retinal pigment epithelial leakage sites, or by photodynamic therapy (PDT) applied to the areas of vessel leakage.
Laser photocoagulation can be performed using different types of lasers such as argon laser, or a newer treatment known as the subthreshold micropulse laser (SML). The objective of performing an argon laser photocoagulation is to create a scar (not too near to the macula) to restore the retinal pigment epithelial barrier in order to prevent further subretinal fluid accumulation. This could also allow the pump function of the surrounding healthy retinal pigment epithelial to return the fluid to the choriocapillaris [17].
SML therapy uses different lasers with varying wavelengths to release a group of molecules known as heat shock proteins [18]. These proteins are involved in repairing retinal pigment epithelial cells and in reducing the inflammation cascade [19].
Injections
Injections applied directly to the eye of anti-VEGF drugs have also been used for the treatment of central serous retinopathy. This group of drugs block vascular leakage and effectively reduces choroidal hyperpermeability. Reports have shown that anti-VEGF injections provide good functional short term outcomes for treating acute and chronic CSR [24], [25].
There are other oral medications that have been prescribed for CSR and they include but not limited to: mineralocorticoid antagonists such as spironolactone and eplerenone and carbonic anhydrase inhibitors such as acetazolamide [10].
It is always the best course of action to consult with an eye health professional on the most appropriate treatment strategy for CSR that caters to each patient’s needs.
DISCLAIMER: THIS WEBSITE DOES NOT PROVIDE MEDICAL ADVICE
The information, including but not limited to, text, graphics, images and other material contained on this website are for informational purposes only. No material on this site is intended to be a substitute for professional medical advice, diagnosis or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
References:
- A. S. Kitzmann, J. S. Pulido, N. N. Diehl, D. O. Hodge, and J. P. Burke, “The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002,” Ophthalmology, vol. 115, no. 1, pp. 169–173, 2008.
- N. K. Sahoo, S. R. Singh, P. Kammari, G. B. Jonnadula, A. V. Das, and J. Chhablani, “Prevalence and Profile of Central Serous Chorioretinopathy in an Indian Cohort,” Nepal. J. Ophthalmol., vol. 11, no. 1, pp. 5–10, 2019.
- A. Kido et al., “Incidence of central serous chorioretinopathy (2011–2018): a nationwide population-based cohort study of Japan,” Br. J. Ophthalmol., p. bjophthalmol-2021-319403, Jul. 2021, doi: 10.1136/bjophthalmol-2021-319403.
- T. H. Rim, H. S. Kim, J. Kwak, J. S. Lee, D. W. Kim, and S. S. Kim, “Association of Corticosteroid Use With Incidence of Central Serous Chorioretinopathy in South Korea,” JAMA Ophthalmol., vol. 136, no. 10, pp. 1164–1169, Oct. 2018, doi: 10.1001/jamaophthalmol.2018.3293.
- D.-C. Tsai et al., “Epidemiology of idiopathic central serous chorioretinopathy in Taiwan, 2001–2006: a population-based study,” PloS One, vol. 8, no. 6, p. e66858, 2013.
- B. N and S. O, “A Review on Imaging Techniques and Algorithms for the Detection of Central serous retinopathy,” in 2021 5th International Conference on Computing Methodologies and Communication (ICCMC), Apr. 2021, pp. 1244–1248. doi: 10.1109/ICCMC51019.2021.9418239.
- R. F. Spaide et al., “Central serous chorioretinopathy in younger and older adults,” Ophthalmology, vol. 103, no. 12, pp. 2070–2080, 1996.
- L. A. Yannuzzi, “Type-A behavior and central serous chorioretinopathy.,” Retina Phila. Pa, vol. 7, no. 2, pp. 111–131, 1987.
- B. Liu, T. Deng, and J. Zhang, “Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis,” Retina, vol. 36, no. 1, pp. 9–19, 2016.
- F. Semeraro et al., “Central serous chorioretinopathy: pathogenesis and management,” Clin. Ophthalmol. Auckl. NZ, vol. 13, p. 2341, 2019.
- Y. Eom, J. Oh, S.-W. Kim, and K. Huh, “Systemic factors associated with central serous chorioretinopathy in Koreans,” Korean J. Ophthalmol., vol. 26, no. 4, pp. 260–264, 2012.
- M. K. Tittl et al., “Systemic findings associated with central serous chorioretinopathy,” Am. J. Ophthalmol., vol. 128, no. 1, pp. 63–68, Jul. 1999, doi: 10.1016/S0002-9394(99)00075-6.
- D. R. Guyer, L. A. Yannuzzi, J. S. Slakter, J. A. Sorenson, A. Ho, and D. Orlock, “Digital indocyanine green videoangiography of central serous chorioretinopathy,” Arch. Ophthalmol., vol. 112, no. 8, pp. 1057–1062, 1994.
- C. Prünte and J. Flammer, “Choroidal capillary and venous congestion in central serous chorioretinopathy,” Am. J. Ophthalmol., vol. 121, no. 1, pp. 26–34, 1996.
- N. V. Baran, V. P. Gürlü, and H. Esgin, “Long‐term macular function in eyes with central serous chorioretinopathy,” Clin. Experiment. Ophthalmol., vol. 33, no. 4, pp. 369–372, 2005.
- R. Liegl and M. W. Ulbig, “Central serous chorioretinopathy,” Ophthalmologica, vol. 232, no. 2, pp. 65–76, 2014.
- M. Spitznas, “Pathogenesis of central serous retinopathy: a new working hypothesis,” Graefes Arch. Clin. Exp. Ophthalmol., vol. 224, no. 4, pp. 321–324, 1986.
- J. K. Luttrull, D. B. Chang, B. W. Margolis, G. Dorin, and D. K. Luttrull, “Laser resensitization of medically unresponsive neovascular age-related macular degeneration: efficacy and implications,” Retina, vol. 35, no. 6, pp. 1184–1194, 2015.
- K. C. Kregel, “Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance,” J. Appl. Physiol., vol. 92, no. 5, pp. 2177–2186, 2002.
- U. Schmidt-Erfurth, S. Michels, I. Barbazetto, and H. Laqua, “Photodynamic effects on choroidal neovascularization and physiological choroid,” Invest. Ophthalmol. Vis. Sci., vol. 43, no. 3, pp. 830–841, 2002.
- W. Chan, D. Lam, T. Lai, B. Tam, D. Liu, and C. Chan, “Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level,” Br. J. Ophthalmol., vol. 87, no. 12, pp. 1453–1458, 2003.
- R. Tzekov et al., “Ocular changes after photodynamic therapy,” Invest. Ophthalmol. Vis. Sci., vol. 47, no. 1, pp. 377–385, 2006.
- F. Semeraro, M. R. Romano, P. Danzi, F. Morescalchi, and C. Costagliola, “Intravitreal bevacizumab versus low-fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy,” Jpn. J. Ophthalmol., vol. 56, no. 6, pp. 608–612, 2012.
- J. W. Lim, M. U. Kim, and M.-C. Shin, “Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy,” Retina, vol. 30, no. 9, pp. 1465–1471, 2010.
- K. B. Schaal, A. E. Hoeh, A. Scheuerle, F. Schuett, and S. Dithmar, “Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy,” Eur. J. Ophthalmol., vol. 19, no. 4, pp. 613–617, 2009.
Tools Designed for Healthier Eyes
Explore our specifically designed products and services backed by eye health professionals to help keep your children safe online and their eyes healthy.
