Choroidal Melanoma
Choroidal melanoma – Written by Dr. Lily Rolfe, BSc, MD and Dr. Roderick O’Day, LLB (Hons), BSc, MBBS (Hons), FRANZCO.
What is choroidal melanoma?
Choroidal melanoma is a rare type of cancer that develops within the eye.
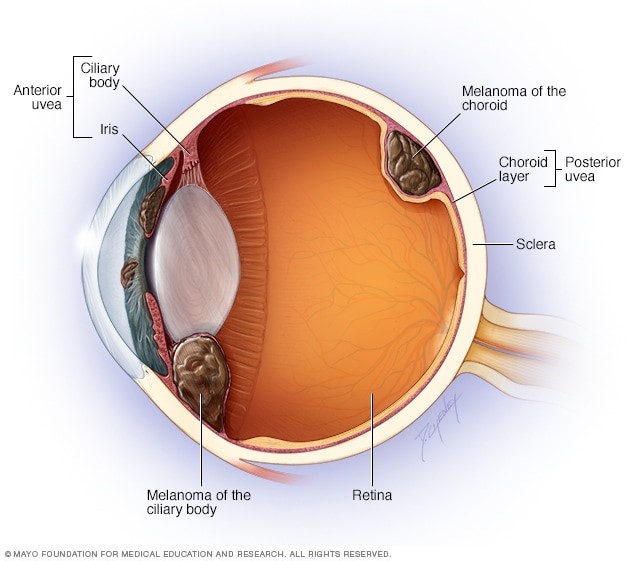
Melanomas may occur at many sites of the body, including the uveal tract of the eye (Figure 1). The uveal tract of the eye includes the iris, ciliary body, and choroid (Figure 2).
The choroid is the most common site for a melanoma in the eye [1]. The choroid is a layer of the eye which contains blood vessels that supply nutrients to the light-sensitive layer of the eye called the retina.
Choroidal melanomas are different to other types of melanomas in both their risk factors and treatment.

This overview will cover the global problem of choroidal melanoma, its risk factors, its common signs and symptoms, how it is diagnosed, and some insights into its management and treatment.
The global problem of choroidal melanoma
While the uveal tract is the second most common site for a melanoma to develop (after the skin), uveal melanomas are rare in general. For example, it is estimated there are only 1500-2000 new cases of uveal melanoma diagnosed in the United States (US) each year [2-4]. Uveal melanomas are more common in Caucasian populations and become more common with age [1, 5, 6]. Males are slightly more likely to be affected by uveal melanoma [1].
Risk factors for choroidal melanoma
The following predisposing factors have been identified which increase the risk of developing uveal melanoma [7, 8]:
- Fair skin colour [1, 5]
- Light eye colour (blue or grey coloured eyes) [8]
- Tendency to get sunburn [9]
- Atypical moles (dysplastic naevi) – unusual-looking moles with irregular microscopic features[10, 11]
- Common skin moles (naevi) and freckles [10]
- Moles in the eye [10, 12]
- Family member with skin melanoma [13-15]
- Family members with uveal melanoma [13-15]
- Oculodermal melanocytosis – a condition present from birth which causes abnormal pigmentation across different areas of the body including the eyes and the skin around the eyes [16, 17]
- Genetic syndromes and mutations
- FAM-M Syndrome [18]
- BAP1 Cancer Predisposition Syndrome [19-21]
- Germline BRCA1/BRCA2 mutations [22-25]
Exposure to sunlight is not a risk factor for uveal melanoma as in the case of skin melanoma [26, 27].
What are the common signs and symptoms of choroidal melanoma?
Choroidal melanomas may or may not be symptomatic depending on their location, size, and activity. In approximately 30% of cases, they are asymptomatic and detected incidentally during an eye examination. When symptoms are present, they may include:
- Blurred vision
- Flashes of light (photopsia)
- Floaters
- Visual field loss
- Pain
- Distorted vision (metamorphopsia) [28]
If you experience any of these signs and symptoms, schedule an appointment with an eye health professional to get your eyes checked. It is also important to note that the development of eye conditions may even start before symptoms appear, which makes going for regular and timely eye checks that much more essential.
How does choroidal melanoma progress?
As choroidal melanomas grow larger, they can cause other problems within the eye. For example, as the tumour grows, it may lift the overlying retina and cause a retinal detachment, which may lead to vision loss.
Other complications include bleeding (haemorrhage) and spreading outside of the eye to other parts of the body (metastasis). The most common site for choroidal melanoma to metastasise is the liver, followed by the lungs [29, 30].
Categories of choroidal melanoma
Choroidal melanomas are commonly classified by their size, which subsequently determines the treatment as the likelihood of metastasis increases with size. Small choroidal melanomas are considered to be less than 3mm in height and/or 10mm in diameter. Medium choroidal melanomas are between 3-8mm in height, and/or less than 16mm in diameter. Large choroidal melanomas are over 8mm in height or 15mm in diameter. As tumour height increases, so too does the mortality rate [31].
How is choroidal melanoma diagnosed?
Choroidal melanomas are diagnosed based on their appearance during a comprehensive eye examination using imaging tools. Choroidal melanomas are distinguished from other lesions in the eye by evaluating its clinical features. The features that confer a higher likelihood of the lesion being a melanoma (as well as an increased risk of growth and metastasis) include:
- Tumour thickness greater than 2mm and diameter greater than 5mm
- Subretinal fluid (i.e. fluid underneath the retina, leaking from the tumour)
- If the lesion is causing reduced vision
- Orange pigment over the surface of the lesion (Figure 3)
- Hollowness on ultrasound imaging [31-33]

While some cancers are diagnosed by taking a tissue sample (biopsy), this is not routinely performed for choroidal melanomas. However, where there is diagnostic uncertainty, a fine-needle aspirate (FNA) biopsy may sometimes be performed. FNA biopsy may be helpful to diagnose suspicious lesions without typical melanoma features, to determine the origin of a cancer (e.g. from the eye itself, or spread from elsewhere in the body) or when visibility is obscured (e.g. by haemorrhage in the eye) and the lesion is unable to be assessed [34, 35].
If you experience any of these signs and symptoms, schedule an appointment with an eye health professional to get your eyes checked. It is also important to note that the development of eye conditions may even start before symptoms appear, which makes going for regular and timely eye checks that much more essential.
Treatment options for choroidal melanoma
The treatment options for choroidal melanoma include laser therapies, radiation, or surgical removal of the eye. The treatment of choice depends on the tumour size, pigment, location, and the presence of any complications.
Radiation therapy
Radiotherapy delivered by a radioactive plaque (brachytherapy) is the most common treatment modality for small and medium-sized melanomas (Figure 4). The radioactive plaque delivers a high radiation dose to the tumour, while minimising collateral damage to the nearby healthy tissue. The plaque is sewn onto the surface of the eye in the operating theatre and left in place for several days before being removed. The dose of radiation and length of time it is left in place depends on the size and location of the tumour, as well as the strength of the radiation.

Other radiation options include external beam radiotherapy, which can be photons or protons. Proton beam radiotherapy, allow for precise high-dose radiation delivery and offer a useful alternative to brachytherapy for tumours close to the optic nerve, but is only available in a few, specialised centres around the world. Photon based external beam radiotherapy, such as Gamma knife, similarly delivers high-precision radiation, and is useful for patients with large melanomas who are seeking to avoid removal of the eye (enucleation) and is more commonly available.
All radiation modalities achieve excellent tumour control and offer long-term survival rates similar to removing the eye [36-42]. Radiotherapy can cause both short-term and long-term complications. Inflammation and irritation of the surface of the eye is commonly seen shortly after radiation treatment, however this usually resolves quickly. While radiation therapy allows a patient to keep their eye and potentially some vision, many patients experience complications which may lead to vision loss and require further surgeries or procedures.
Delayed complications can present in the months to years following initial treatment. Radiation may lead to vision loss through several mechanisms, including cataract, damage to the blood vessels in the eye (radiation retinopathy), optic nerve damage (optic neuropathy), or by stimulating the formation of immature, leaky blood vessels (neovascularisation) throughout the eye which can lead to glaucoma. Less commonly, radiation therapies may lead to retinal detachment and melting of the outer layer of the eye (scleral melt) [43-45].
Surgery
Before brachytherapy, enucleation was the mainstay of treatment for all choroidal melanomas. Today, enucleation is primarily for tumours too large for brachytherapy, for tumours that recur following brachytherapy, for eyes that have become blind or painful following complications, or if the patient chooses it [46]. A spherical implant is inserted into the eye socket under the remaining tissues of the eye. A prosthesis is typically made in the weeks to months after surgery to create the appearance of a normal eye.
Another surgical option involves cutting the tumour out of the eye, either accessed externally through an opening created in the outer layer of the eye (exoresection) or via passing a cutter inside the eye (endoresection). This procedure is only performed at some ocular oncology centres, and sometimes only after radiotherapy. Endo- and exoresection are associated with complications such as retinal detachment, bleeding and tumour recurrence [47-50].
In severe cases where the tumour has spread outside of the eye into the eye socket, surgery to removal of the entire contents of the eye socket (exenteration) may be required.
Laser therapy
Laser therapies offer a non-invasive out-of-hospital treatment option for small choroidal melanomas, including photodynamic therapy or transpupillary thermotherapy. Photodynamic therapy involves administering a light-sensitive dye and laser to induce tumour cell destruction, and is most effective in small, non-pigmented (amelanotic) tumours [51-56]. Transpupillary thermotherapy utilises a heating laser which aims to destroy the tumour’s vascular supply, and is most useful for small, pigmented choroidal melanomas [57, 58].
Both photodynamic therapy and transpupillary thermotherapy have been associated with visually significant complications [59-65]. However, the relatively high recurrence rate precludes the use of laser therapies as a standalone treatment – but they may be used in conjunction with radiotherapy to consolidate treatment [52, 55, 56, 66].
Ongoing management of choroidal melanoma
After initial treatment has been undertaken, regular follow-up is required for many years afterwards. The follow-up appointments involve screening for local recurrence of the tumour (in and around the eye) as well as metastatic spread (most commonly to the liver) and managing any complications of treatment.
Local recurrence is assessed by examination of the eye and ultrasound imaging of the eye. Metastatic spread is evaluated by blood tests to evaluate liver function as well as liver imaging. Liver imaging may involve ultrasound, computerised tomography (CT) scans or magnetic resonance imaging (MRI). If metastatic spread has occurred, patients may undergo systemic chemotherapy or surgery to remove liver tumours.
Treatment of metastatic choroidal melanoma is very difficult and the prognosis is, generally, poor. This is an area of active research into therapies that may potentially improve a patient’s survival, with a number of drugs being assessed currently. Involvement of medical oncologists who specialise in this area is preferable if it is available.
Can choroidal melanoma be prevented?
Currently, there is no effective way to prevent a choroidal melanoma from developing. Regular eye examinations are of great importance to identify tumours in their earlier, asymptomatic, and smaller stages, which carry a lower risk of metastasis and require less invasive treatment.
DISCLAIMER: THIS WEBSITE DOES NOT PROVIDE MEDICAL ADVICE
The information, including but not limited to, text, graphics, images and other material contained on this website are for informational purposes only. No material on this site is intended to be a substitute for professional medical advice, diagnosis or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
References
- M. E. Aronow, A. K. Topham, and A. D. Singh, “Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973-2013),” Ocul Oncol Pathol, vol. 4, no. 3, pp. 145-151, Apr 2018, doi: 10.1159/000480640.
- S. Kaliki and C. L. Shields, “Uveal melanoma: relatively rare but deadly cancer,” Eye (Lond), vol. 31, no. 2, pp. 241-257, Feb 2017, doi: 10.1038/eye.2016.275.
- C. C. McLaughlin, X. C. Wu, A. Jemal, H. J. Martin, L. M. Roche, and V. W. Chen, “Incidence of noncutaneous melanomas in the U.S,” Cancer, vol. 103, no. 5, pp. 1000-7, Mar 1 2005, doi: 10.1002/cncr.20866.
- A. D. Singh, M. E. Turell, and A. K. Topham, “Uveal melanoma: trends in incidence, treatment, and survival,” Ophthalmology, vol. 118, no. 9, pp. 1881-5, Sep 2011, doi: 10.1016/j.ophtha.2011.01.040.
- D. N. Hu, G. P. Yu, S. A. McCormick, S. Schneider, and P. T. Finger, “Population-based incidence of uveal melanoma in various races and ethnic groups,” Am J Ophthalmol, vol. 140, no. 4, pp. 612-7, Oct 2005, doi: 10.1016/j.ajo.2005.05.034.
- A. D. Singh and A. Topham, “Incidence of uveal melanoma in the United States: 1973-1997,” Ophthalmology, vol. 110, no. 5, pp. 956-61, May 2003, doi: 10.1016/S0161-6420(03)00078-2.
- T. Nayman, C. Bostan, P. Logan, and M. N. Burnier, Jr., “Uveal Melanoma Risk Factors: A Systematic Review of Meta-Analyses,” Curr Eye Res, vol. 42, no. 8, pp. 1085-1093, Aug 2017, doi: 10.1080/02713683.2017.1297997.
- M. A. Saornil, “Iris colour and uveal melanoma,” Can J Ophthalmol, vol. 39, no. 4, pp. 448-52, Jun 2004, doi: 10.1016/s0008-4182(04)80018-8.
- E. Weis, C. P. Shah, M. Lajous, J. A. Shields, and C. L. Shields, “The association between host susceptibility factors and uveal melanoma: a meta-analysis,” Arch Ophthalmol, vol. 124, no. 1, pp. 54-60, Jan 2006, doi: 10.1001/archopht.124.1.54.
- V. Bataille, P. Sasieni, J. Cuzick, J. L. Hungerford, A. Swerdlow, and J. A. Bishop, “Risk of ocular melanoma in relation to cutaneous and iris naevi,” Int J Cancer, vol. 60, no. 5, pp. 622-6, Mar 3 1995, doi: 10.1002/ijc.2910600509.
- H. Hammer, J. Olah, and E. Toth-Molnar, “Dysplastic nevi are a risk factor for uveal melanoma,” Eur J Ophthalmol, vol. 6, no. 4, pp. 472-4, Oct-Dec 1996. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/8997595.
- J. W. Harbour, M. A. Brantley, Jr., H. Hollingsworth, and M. Gordon, “Association between posterior uveal melanoma and iris freckles, iris naevi, and choroidal naevi,” Br J Ophthalmol, vol. 88, no. 1, pp. 36-8, Jan 2004, doi: 10.1136/bjo.88.1.36.
- M. H. Abdel-Rahman, R. Pilarski, S. Ezzat, J. Sexton, and F. H. Davidorf, “Cancer family history characterization in an unselected cohort of 121 patients with uveal melanoma,” Fam Cancer, vol. 9, no. 3, pp. 431-8, Sep 2010, doi: 10.1007/s10689-010-9328-7.
- A. D. Singh et al., “Familial uveal melanoma. Clinical observations on 56 patients,” Arch Ophthalmol, vol. 114, no. 4, pp. 392-9, Apr 1996, doi: 10.1001/archopht.1996.01100130388005.
- C. L. van Hees, M. J. Jager, J. C. Bleeker, H. Kemme, and W. Bergman, “Occurrence of cutaneous and uveal melanoma in patients with uveal melanoma and their first degree relatives,” Melanoma Res, vol. 8, no. 2, pp. 175-80, Apr 1998, doi: 10.1097/00008390-199804000-00013.
- A. D. Singh, P. De Potter, B. A. Fijal, C. L. Shields, J. A. Shields, and R. C. Elston, “Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis,” Ophthalmology, vol. 105, no. 1, pp. 195-8, Jan 1998, doi: 10.1016/s0161-6420(98)92205-9.
- C. L. Shields et al., “Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes,” JAMA Ophthalmol, vol. 131, no. 8, pp. 993-1003, Aug 2013, doi: 10.1001/jamaophthalmol.2013.129.
- A. D. Singh, C. L. Shields, J. A. Shields, R. C. Eagle, and P. De Potter, “Uveal melanoma and familial atypical mole and melanoma (FAM-M) syndrome,” Ophthalmic Genet, vol. 16, no. 2, pp. 53-61, Jun 1995, doi: 10.3109/13816819509056913.
- C. N. Njauw et al., “Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families,” PLoS One, vol. 7, no. 4, p. e35295, 2012, doi: 10.1371/journal.pone.0035295.
- T. Wiesner et al., “Germline mutations in BAP1 predispose to melanocytic tumors,” Nat Genet, vol. 43, no. 10, pp. 1018-21, Aug 28 2011, doi: 10.1038/ng.910.
- J. W. Harbour et al., “Frequent mutation of BAP1 in metastasizing uveal melanomas,” Science, vol. 330, no. 6009, pp. 1410-3, Dec 3 2010, doi: 10.1126/science.1194472.
- C. Cruz, A. Teule, J. M. Caminal, I. Blanco, and J. M. Piulats, “Uveal melanoma and BRCA1/BRCA2 genes: a relationship that needs further investigation,” J Clin Oncol, vol. 29, no. 34, pp. e827-9, Dec 1 2011, doi: 10.1200/JCO.2011.37.8828.
- D. F. Easton et al., “Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13,” Am J Hum Genet, vol. 61, no. 1, pp. 120-8, Jul 1997, doi: 10.1086/513891.
- J. Iscovich, M. Abdulrazik, C. Cour, A. Fischbein, J. Pe’er, and D. E. Goldgar, “Prevalence of the BRCA2 6174 del T mutation in Israeli uveal melanoma patients,” Int J Cancer, vol. 98, no. 1, pp. 42-4, Mar 1 2002, doi: 10.1002/ijc.10155.
- O. M. Sinilnikova et al., “Germline brca2 sequence variants in patients with ocular melanoma,” Int J Cancer, vol. 82, no. 3, pp. 325-8, Jul 30 1999, doi: 10.1002/(sici)1097-0215(19990730)82:3<325::aid-ijc3>3.0.co;2-g.
- A. D. Singh, I. G. Rennie, S. Seregard, M. Giblin, and J. McKenzie, “Sunlight exposure and pathogenesis of uveal melanoma,” Surv Ophthalmol, vol. 49, no. 4, pp. 419-28, Jul-Aug 2004, doi: 10.1016/j.survophthal.2004.04.009.
- C. P. Shah, E. Weis, M. Lajous, J. A. Shields, and C. L. Shields, “Intermittent and chronic ultraviolet light exposure and uveal melanoma: a meta-analysis,” Ophthalmology, vol. 112, no. 9, pp. 1599-607, Sep 2005, doi: 10.1016/j.ophtha.2005.04.020.
- E. M. Damato and B. E. Damato, “Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients,” Ophthalmology, vol. 119, no. 8, pp. 1582-9, Aug 2012, doi: 10.1016/j.ophtha.2012.01.048.
- L. Cerbone et al., “Clinical presentation, pathological features and natural course of metastatic uveal melanoma, an orphan and commonly fatal disease,” Oncology, vol. 86, no. 3, pp. 185-9, 2014, doi: 10.1159/000358729.
- “Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group,” Arch Ophthalmol, vol. 115, no. 7, pp. 886-93, Jul 1997. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/9230829.
- “Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group,” Arch Ophthalmol, vol. 115, no. 12, pp. 1537-44, Dec 1997, doi: 10.1001/archopht.1997.01100160707007.
- C. L. Shields, J. A. Shields, H. Kiratli, P. De Potter, and J. R. Cater, “Risk factors for growth and metastasis of small choroidal melanocytic lesions,” Ophthalmology, vol. 102, no. 9, pp. 1351-61, Sep 1995. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/9097773.
- C. L. Shields, J. Cater, J. A. Shields, A. D. Singh, M. C. Santos, and C. Carvalho, “Combination of clinical factors predictive of growth of small choroidal melanocytic tumors,” Arch Ophthalmol, vol. 118, no. 3, pp. 360-4, Mar 2000, doi: 10.1001/archopht.118.3.360.
- N. Eide and L. Walaas, “Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review,” Acta Ophthalmol, vol. 87, no. 6, pp. 588-601, Sep 2009, doi: 10.1111/j.1755-3768.2009.01637.x.
- A. D. Singh and C. V. Biscotti, “Fine needle aspiration biopsy of ophthalmic tumors,” Saudi J Ophthalmol, vol. 26, no. 2, pp. 117-23, Apr 2012, doi: 10.1016/j.sjopt.2012.01.005.
- G. Collaborative Ocular Melanoma Study, “The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28,” Arch Ophthalmol, vol. 124, no. 12, pp. 1684-93, Dec 2006, doi: 10.1001/archopht.124.12.1684.
- M. Diener-West et al., “The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18,” Arch Ophthalmol, vol. 119, no. 7, pp. 969-82, Jul 2001, doi: 10.1001/archopht.119.7.969.
- J. C. Wen, S. C. Oliver, and T. A. McCannel, “Ocular complications following I-125 brachytherapy for choroidal melanoma,” Eye (Lond), vol. 23, no. 6, pp. 1254-68, Jun 2009, doi: 10.1038/eye.2009.43.
- M. J. Abrams, N. L. Gagne, C. S. Melhus, and J. E. Mignano, “Brachytherapy vs. external beam radiotherapy for choroidal melanoma: Survival and patterns-of-care analyses,” Brachytherapy, vol. 15, no. 2, pp. 216-23, Mar-Apr 2016, doi: 10.1016/j.brachy.2015.12.001.
- M. J. Sikuade, S. Salvi, P. A. Rundle, D. G. Errington, A. Kacperek, and I. G. Rennie, “Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma,” Eye (Lond), vol. 29, no. 9, pp. 1194-8, Sep 2015, doi: 10.1038/eye.2015.109.
- B. Damato, A. Kacperek, M. Chopra, I. R. Campbell, and R. D. Errington, “Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience,” Int J Radiat Oncol Biol Phys, vol. 62, no. 5, pp. 1405-11, Aug 1 2005, doi: 10.1016/j.ijrobp.2005.01.016.
- D. C. Weber et al., “Proton beam radiotherapy versus fractionated stereotactic radiotherapy for uveal melanomas: A comparative study,” Int J Radiat Oncol Biol Phys, vol. 63, no. 2, pp. 373-84, Oct 1 2005, doi: 10.1016/j.ijrobp.2005.01.057.
- E. Gragoudas, W. Li, M. Goitein, A. M. Lane, J. E. Munzenrider, and K. M. Egan, “Evidence-based estimates of outcome in patients irradiated for intraocular melanoma,” Arch Ophthalmol, vol. 120, no. 12, pp. 1665-71, Dec 2002, doi: 10.1001/archopht.120.12.1665.
- B. M. Melia et al., “Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16,” Ophthalmology, vol. 108, no. 2, pp. 348-66, Feb 2001, doi: 10.1016/s0161-6420(00)00526-1.
- P. K. Lommatzsch, C. Werschnik, and E. Schuster, “Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma,” Graefes Arch Clin Exp Ophthalmol, vol. 238, no. 2, pp. 129-37, Feb 2000, doi: 10.1007/pl00007880.
- R. W. Milam and A. B. Daniels, “Uveal Melanoma,” Springer International Publishing, 2018, pp. 273-312.
- B. Damato, “The role of eyewall resection in uveal melanoma management,” Int Ophthalmol Clin, vol. 46, no. 1, pp. 81-93, Winter 2006, doi: 10.1097/01.iio.0000195862.71558.c8.
- T. Kivela, I. Puusaari, and B. Damato, “Transscleral resection versus iodine brachytherapy for choroidal malignant melanomas 6 millimeters or more in thickness: a matched case-control study,” Ophthalmology, vol. 110, no. 11, pp. 2235-44, Nov 2003, doi: 10.1016/j.ophtha.2003.02.001.
- N. E. Bechrakis and M. H. Foerster, “Neoadjuvant proton beam radiotherapy combined with subsequent endoresection of choroidal melanomas,” Int Ophthalmol Clin, vol. 46, no. 1, pp. 95-107, Winter 2006, doi: 10.1097/01.iio.0000195856.31324.00.
- L. Konstantinidis, C. Groenewald, S. E. Coupland, and B. Damato, “Long-term outcome of primary endoresection of choroidal melanoma,” Br J Ophthalmol, vol. 98, no. 1, pp. 82-5, Jan 2014, doi: 10.1136/bjophthalmol-2013-304022.
- R. R. Allison and K. Moghissi, “Photodynamic Therapy (PDT): PDT Mechanisms,” Clin Endosc, vol. 46, no. 1, pp. 24-9, Jan 2013, doi: 10.5946/ce.2013.46.1.24.
- W. G. Campbell and T. M. Pejnovic, “Treatment of amelanotic choroidal melanoma with photodynamic therapy,” Retina, vol. 32, no. 7, pp. 1356-62, Jul 2012, doi: 10.1097/IAE.10.1097/IAE.0b013e31822c28ec.
- M. R. Hamblin and E. L. Newman, “On the mechanism of the tumour-localising effect in photodynamic therapy,” J Photochem Photobiol B, vol. 23, no. 1, pp. 3-8, Apr 1994, doi: 10.1016/s1011-1344(94)80018-9.
- G. Jori and E. Reddi, “The role of lipoproteins in the delivery of tumour-targeting photosensitizers,” Int J Biochem, vol. 25, no. 10, pp. 1369-75, Oct 1993, doi: 10.1016/0020-711x(93)90684-7.
- R. F. O’Day, T. M. Pejnovic, T. Isaacs, J. S. Muecke, W. J. Glasson, and W. G. Campbell, “Australian and New Zealand Study of Photodynamic Therapy in Choroidal Amelanotic Melanoma,” Retina, vol. 40, no. 5, pp. 972-976, May 2020, doi: 10.1097/IAE.0000000000002520.
- P. Rundle, “Treatment of posterior uveal melanoma with multi-dose photodynamic therapy,” Br J Ophthalmol, vol. 98, no. 4, pp. 494-7, Apr 2014, doi: 10.1136/bjophthalmol-2013-304432.
- B. Fuisting and G. Richard, “Transpupillary thermotherapy (TTT) – Review of the clinical indication spectrum ” Medical Laser Application, vol. 25, no. 4, pp. 214-222, 2010.
- O. P. Garcia, P. R. M. Lyra, A. Fernandes, R. d. Cassia, and F. d. Lima, “The influence of the vitreous humor viscosity during laser-induced thermal damage in choroidal melanomas ” International Journal of Thermal Sciences, vol. 136, pp. 444-456, 2019.
- E. Cerman and O. Cekic, “Clinical use of photodynamic therapy in ocular tumors,” Surv Ophthalmol, vol. 60, no. 6, pp. 557-74, Nov-Dec 2015, doi: 10.1016/j.survophthal.2015.05.004.
- D. G. Godfrey, R. G. Waldron, and A. Capone, Jr., “Transpupillary thermotherapy for small choroidal melanoma,” Am J Ophthalmol, vol. 128, no. 1, pp. 88-93, Jul 1999, doi: 10.1016/s0002-9394(99)00063-x.
- J. W. Harbour, T. A. Meredith, P. A. Thompson, and M. E. Gordon, “Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas,” Ophthalmology, vol. 110, no. 11, pp. 2207-14; discussion 2215, Nov 2003, doi: 10.1016/S0161-6420(03)00858-3.
- J. A. Oosterhuis, H. G. Journee-de Korver, H. M. Kakebeeke-Kemme, and J. C. Bleeker, “Transpupillary thermotherapy in choroidal melanomas,” Arch Ophthalmol, vol. 113, no. 3, pp. 315-21, Mar 1995, doi: 10.1001/archopht.1995.01100030071024.
- C. L. Shields and J. A. Shields, “Transpupillary thermotherapy for choroidal melanoma,” Curr Opin Ophthalmol, vol. 10, no. 3, pp. 197-203, Jun 1999, doi: 10.1097/00055735-199906000-00008.
- C. L. Shields, J. A. Shields, P. DePotter, and S. Kheterpal, “Transpupillary thermotherapy in the management of choroidal melanoma,” Ophthalmology, vol. 103, no. 10, pp. 1642-50, Oct 1996, doi: 10.1016/s0161-6420(96)30451-x.
- B. M. Stoffelns, “Primary transpupillary thermotherapy (TTT) for malignant choroidal melanoma,” Acta Ophthalmol Scand, vol. 80, no. 1, pp. 25-31, Feb 2002, doi: 10.1034/j.1600-0420.2002.800106.x.
- A. Mashayekhi et al., “Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: importance of risk factors in tumor control,” Ophthalmology, vol. 122, no. 3, pp. 600-9, Mar 2015, doi: 10.1016/j.ophtha.2014.09.029.
- C. L. Shields et al., “White Paper on Ophthalmic Imaging for Choroidal Nevus Identification and Transformation into Melanoma,” Translational Vision Science & Technology, vol. 10, no. 2, p. 24, 2021, doi: 10.1167/tvst.10.2.24.
Tools Designed for Healthier Eyes
Explore our specifically designed products and services backed by eye health professionals to help keep your children safe online and their eyes healthy.
