Leber hereditary optic neuropathy
Leber hereditary optic neuropathy – Written by Dr. Isabel Lopez Sanchez, Ph.D.
What is Leber hereditary optic neuropathy?
Leber hereditary optic neuropathy (LHON) is a rare genetic disease that can cause sudden and permanent vision loss. LHON is named after Dr Theodore Leber, a German ophthalmologist who first reported the condition in 1871, and should not be confused with Leber congenital amaurosis.
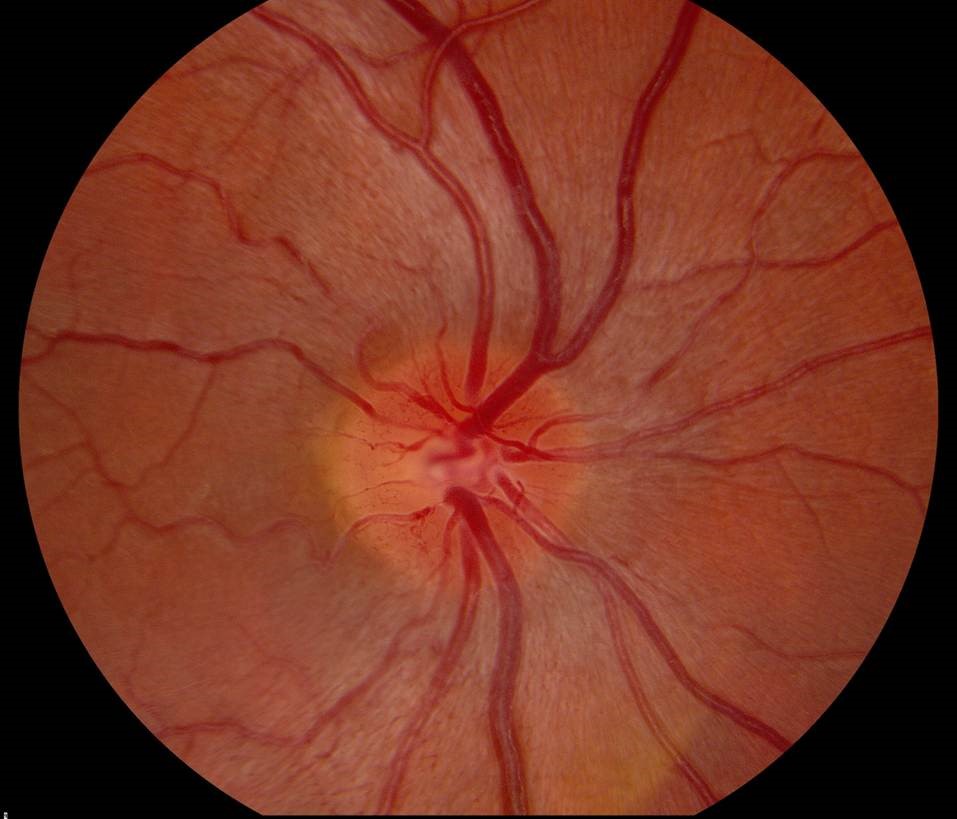
LHON is characterised by the loss of retinal ganglion cells in the retina, the neurons whose axons form the optic nerve. The disease is caused by mutations in mitochondrial DNA genes. The vast majority of people affected by LHON carry one of three genetic mutations: m.11778G>A (MT-ND4), m.14484T>C (MT-ND6) or m.3460G>A (MT-ND1). Other rare mutations are responsible for a small number of cases. LHON affects predominantly young men aged 15 to 30 years old. However, women, older adults, and children are also at risk.
This overview will cover how many people are affected by LHON, its common signs and symptoms, it causes and risk factors, how it is diagnosed, as well as its treatment options and methods of prevention.
How many people are affected by Leber hereditary optic neuropathy?
The presence of a LHON mutation is not in itself sufficient to cause vision loss, and most people with a LHON mutation preserve good vision throughout their lives. It is not known how many people are affected by vision loss due to LHON worldwide.
Studies in Finland, Denmark, North-East England, and Australia indicate that between 1 in 31,000 to 1 in 68,000 individuals within the total population of those countries are affected by vision loss due to LHON [1-4]. However, LHON mutations are relatively common and estimated to be present in 1 in 10,000 individuals [3].
Males are at a higher risk of losing vision due to LHON than females. Population studies have shown a risk of vision loss of 20 to 50% for males and 4 to 28% for females with a LHON mutation [1-3]. Research suggests that oestrogen may protect women from developing vision loss [5].
What causes Leber hereditary optic neuropathy?
LHON is a mitochondrial genetic disease and therefore has a mitochondrial pattern of inheritance. Mitochondrial DNA is contained within mitochondria, rather than the nucleus of the cell, where most of our genes are found.
Mitochondrial DNA is inherited exclusively from a person’s mother. For this reason, a mother carrying a LHON mutation will pass it on to all her children, males and females, and the female offspring will in turn pass it on to their children in future generations. Fathers who carry a LHON mutation do not pass it on to their children, even if they show symptoms of the disease.
It is important to note that all women with a LHON mutation, even those who do not have any signs or symptoms of the disease, will pass on the mutation to their children. However, the presence of a LHON mutation does not necessarily mean a person will lose vision.
What are the risk factors of Leber hereditary optic neuropathy?
The presence of a LHON mutation is not in itself sufficient to cause vision loss. Studies have indicated that environmental risk factors including smoking, heavy alcohol consumption and exposure to toxic drugs may trigger vision loss in some individuals with a LHON mutation [6-8]. For this reason, individuals who carry a LHON mutation should avoid smoking, moderate their alcohol intake, and avoid binge-drinking.
Additional environmental triggers for vision loss, including head trauma, exposure to industrial toxins, and medications that are toxic to the mitochondria should also be avoided [9]. Additional genetic risk factors are suspected but are currently unknown.
What is “Leber hereditary optic neuropathy Plus”?
Vision loss is typically the only symptom of LHON. However, some individuals develop additional neurological symptoms, including movement disorders, tremors, and multiple sclerosis-like features. In these individuals, the disease is described as “LHON Plus”.
What are the common signs and symptoms of Leber hereditary optic neuropathy?

LHON symptoms usually begin as sudden and painless central scotoma (loss of central vision). In most cases, vision loss occurs in one eye, followed by vision loss in the second eye within a year. Over time, vision in both eyes worsen and colour vision is also impaired.
Although LHON does not cause total blindness, central vision loss is severe and reaches the threshold for legal blindness (20/200). Vision loss is permanent in most cases, although spontaneous recovery has been reported in some cases, particularly amongst young patients with the m.14484T>C mutation.
People affected by LHON are unable to read standard-sized text, recognise faces, and drive. However, because peripheral vision remains intact, individuals are able to carry out tasks independently, such as walking. Assistance, including a guide dog or a cane are often required for safe mobility.
f you experience any of these signs and symptoms, schedule an appointment with an eye health professional to get your eyes checked. It is also important to note that the development of eye conditions may even start before symptoms appear, which makes going for regular and timely eye checks that much more essential.
How is Leber hereditary optic neuropathy diagnosed?
Individuals who do not have a family history of LHON often face lengthy delays in diagnosis. Because LHON is a rare disease, most clinicians, including ophthalmologists, will not consider LHON as a possible cause of unexplained loss of central vision, and will instead suspect optic neuritis — inflammation of the optic nerve. However, unlike LHON, optic neuritis is associated with pain, affects only one eye, and is temporary.
Visual tests
Visual tests may include visual field, electroretinogram and examination of the optic discs. Additionally, magnetic resonance imaging (MRI), computed tomography (CT), and lumbar puncture may be conducted to rule out other possible causes of vision loss such as the presence of a brain tumour or infection in the central nervous system.
Molecular genetic tests
A LHON diagnosis is established in a person with ocular manifestations of LHON and by the identification of a LHON mutation during molecular genetic testing. If LHON is suspected, DNA from a blood or saliva sample can be used for genetic testing by an accredited laboratory or specialised research centre. Most laboratories only test for the presence of the three most common LHON mutations — m.11778G>A, m.14484T>C or m.3460G>A — and individuals who have other less common LHON mutations will require additional testing, including full mitochondrial DNA sequencing.
Because we know the maternal pattern of inheritance of LHON, all maternally related individuals of a family member with a LHON mutation will also have the mutation. Targeted genetic screening can be conducted if desired, to confirm the presence of a LHON mutation in a maternal relative.
In families with a known LHON mutation, sudden and unexplained vision loss will likely be attributed to LHON. It is critical to note that a LHON mutation does not necessarily cause vision loss, and most people with a LHON mutation will not lose vision. Genetic counselling is highly recommended for individuals with a genetic diagnosis of LHON, even in the absence of symptoms.
What are the treatment options for Leber hereditary optic neuropathy?
In most countries, there are no approved treatments for LHON. Idebenone (Raxone®) is currently the only medicine approved by the European Medicines Agency for the treatment of LHON. Studies so far indicate visual improvements in some patients treated with idebenone, especially amongst those treated within the first year of symptoms onset [10].
Clinical management is mainly supportive, including nutritional supplements, low vision aids, and healthy lifestyle changes. Individuals with extra-ocular symptoms or “LHON Plus” will require additional specialist support to manage their neurological symptoms. Some of the experimental therapies for LHON include the following.
Gene therapy
Several gene therapy clinical trials have shown promising results in individuals with the m.11778G>A LHON mutation. Gene therapy aims to replace the mutated gene with a healthy version of the same gene to prevent disease or improve symptoms. This approach involves an injection directly into the vitreous, the jelly-like space in the back of the eye, of adeno-associated virus 2 (AAV2).
AAV2 is used as a genetic vehicle to carry a healthy replacement for the mutated gene. Individuals treated this way have shown significant visual improvements [11]. However, gene therapy in LHON is still at the experimental stage and additional research is required to determine optimal treatment conditions. For additional information see ClinicalTrials.gov.
Hormone replacement therapy
A single study suggested that hormone replacement therapy may protect women with a LHON mutation from vision loss [12]. This suggests hormone replacement therapy under medical supervision and close monitoring may be beneficial, in particular for menopausal women who carry a LHON mutation.
Stem cell therapies
There are currently no approved stem cell therapies for the treatment of LHON, and unproven stem cell “treatments” should be avoided as they can be unsafe. The Stem Cell Ophthalmology Treatment Study is the only clinical trial thus far to study the use of bone marrow-derived stem cells in retinal and optic nerve diseases, including LHON [13]. This study showed improvement in visual acuity and peripheral vision in five LHON patients. For additional information see ClinicalTrials.gov. Additional research is necessary to determine the future beneficial potential of stem cell therapy in LHON.
What additional support is available for people with Leber hereditary optic neuropathy?
LHON community online resources provide psychosocial support and valuable practical information for people affected by this disease, including peer support, assistive technology, mobility, education, and employment. Notable online resources include lhon.org (United States) and LHONsociety.org (United Kingdom).
Can I prevent Leber hereditary optic neuropathy?
Currently, the only way to prevent vision loss due to LHON is through the use of in vitro fertilisation (IVF) techniques aimed at preventing the transmission of mitochondrial DNA mutations from the mother to the unborn child. This approach is referred to as ‘Mitochondrial Donation’.
Mitochondrial Donation involves the use of a donated egg with healthy mitochondria to replace the mother’s mutated mitochondria, before or after fertilisation of the egg by the father’s sperm. Because of the contribution of a donor egg, Mitochondrial Donation is also known as ‘Three parent IVF’. Mitochondrial donation has only been approved in the United Kingdom. Legal and ethical implications of the use of this IVF technique are being debated worldwide.
DISCLAIMER: THIS WEBSITE DOES NOT PROVIDE MEDICAL ADVICE
The information, including but not limited to, text, graphics, images and other material contained on this website are for informational purposes only. No material on this site is intended to be a substitute for professional medical advice, diagnosis or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or treatment and before undertaking a new healthcare regimen, and never disregard professional medical advice or delay in seeking it because of something you have read on this website.
References
- A. Puomila et al., “Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland,” Eur J Hum Genet, vol. 15, no. 10, pp. 1079-89, Oct 2007, doi: 10.1038/sj.ejhg.5201828.
- D. A. Mackey and R. G. Buttery, “Leber hereditary optic neuropathy in Australia,” Australian and New Zealand journal of ophthalmology, vol. 20, no. 3, pp. 177-84, Aug 1992, doi: 10.1111/j.1442-9071.1992.tb00937.x.
- P. Y. Man, P. G. Griffiths, D. T. Brown, N. Howell, D. M. Turnbull, and P. F. Chinnery, “The epidemiology of Leber hereditary optic neuropathy in the North East of England,” American journal of human genetics, vol. 72, no. 2, pp. 333-9, Feb 2003, doi: 10.1086/346066.
- T. Rosenberg et al., “Prevalence and Genetics of Leber Hereditary Optic Neuropathy in the Danish Population,” (in eng), Investigative ophthalmology & visual science, vol. 57, no. 3, pp. 1370-5, Mar 2016, doi: 10.1167/iovs.15-18306.
- C. Giordano et al., “Oestrogens ameliorate mitochondrial dysfunction in Leber’s hereditary optic neuropathy,” Brain : a journal of neurology, vol. 134, no. Pt 1, pp. 220-34, Jan 2011, doi: 10.1093/brain/awq276.
- M. A. Kirkman et al., “Gene-environment interactions in Leber hereditary optic neuropathy,” (in eng), Brain, vol. 132, no. Pt 9, pp. 2317-26, Sep 2009, doi: 10.1093/brain/awp158.
- D. A. Mackey et al., “Leber’s hereditary optic neuropathy triggered by antiretroviral therapy for human immunodeficiency virus,” (in eng), Eye (Lond), vol. 17, no. 3, pp. 312-7, Apr 2003, doi: 10.1038/sj.eye.6700362.
- J. H. Seo, J. M. Hwang, and S. S. Park, “Antituberculosis medication as a possible epigenetic factor of Leber’s hereditary optic neuropathy,” (in eng), Clin Exp Ophthalmol, vol. 38, no. 4, pp. 363-6, May 2010, doi: 10.1111/j.1442-9071.2010.02240.x.
- K. Kogachi, A. Ter-Zakarian, S. Asanad, A. Sadun, and R. Karanjia, “Toxic medications in Leber’s hereditary optic neuropathy,” (in eng), Mitochondrion, vol. 46, pp. 270-277, May 2019, doi: 10.1016/j.mito.2018.07.007.
- C. B. Catarino et al., “Real-World Clinical Experience With Idebenone in the Treatment of Leber Hereditary Optic Neuropathy,” Journal of Neuro-Ophthalmology, vol. 40, no. 4, 2020. [Online]. Available: https://journals.lww.com/jneuro-ophthalmology/Fulltext/2020/12000/Real_World_Clinical_Experience_With_Idebenone_in.20.aspx.
- N. J. Newman et al., “Efficacy and Safety of Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy Treated within 6 Months of Disease Onset,” (in eng), Ophthalmology, vol. 128, no. 5, pp. 649-660, May 2021, doi: 10.1016/j.ophtha.2020.12.012.
- M. Fantini, S. Asanad, R. Karanjia, and A. Sadun, “Hormone replacement therapy in Leber’s hereditary optic neuropathy: Accelerated visual recovery in vivo,” (in eng), J Curr Ophthalmol, vol. 31, no. 1, pp. 102-105, Mar 2019, doi: 10.1016/j.joco.2018.10.003.
- J. N. Weiss, S. Levy, and S. C. Benes, “Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow-derived stem cells in the treatment of Leber’s hereditary optic neuropathy,” (in eng), Neural Regen Res, vol. 11, no. 10, pp. 1685-1694, 2016, doi: 10.4103/1673-5374.193251.
Tools Designed for Healthier Eyes
Explore our specifically designed products and services backed by eye health professionals to help keep your children safe online and their eyes healthy.
